Energy at low voltage in alkaline batteries

When looking at discharge curves for Alkaline batteries they always stops at 0.8 volt, why not showing down to 0 to get all energy?
There are two reasons that is usual stated:
- There is very little energy below 0.8 volt.
- Equipment will usual not work below 0.8 volt.
For these curves I have used constant power discharge, this matches how a lot of equipment draws power. It has an internal circuit that will boost the battery voltage to the actual working voltage, this means the power is constant and the current will be increasing when the voltage drops.
In fact, my curves are a bit conversative, usual the efficiency of a boost converter will drop at lower voltages, i.e. the device will require even more power/current that my charts shows.
All curves with the same discharge power is based on the same test data, it is just different ways to present the data.
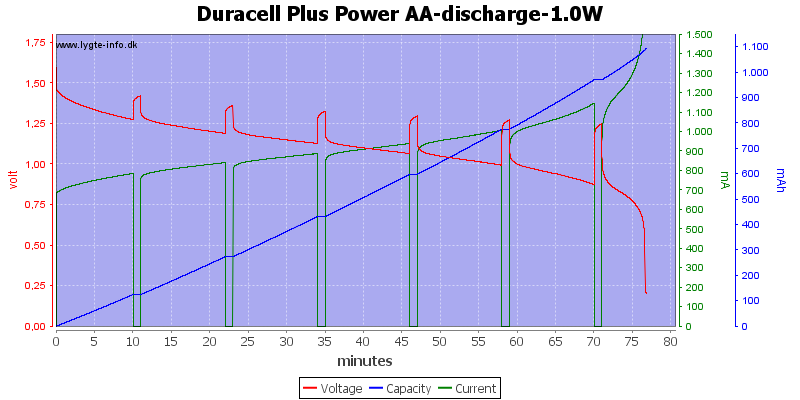
Test with 1 watt discharge
1 watt is 0.667A at 1.5 volt, but current will increase as the voltage drops.
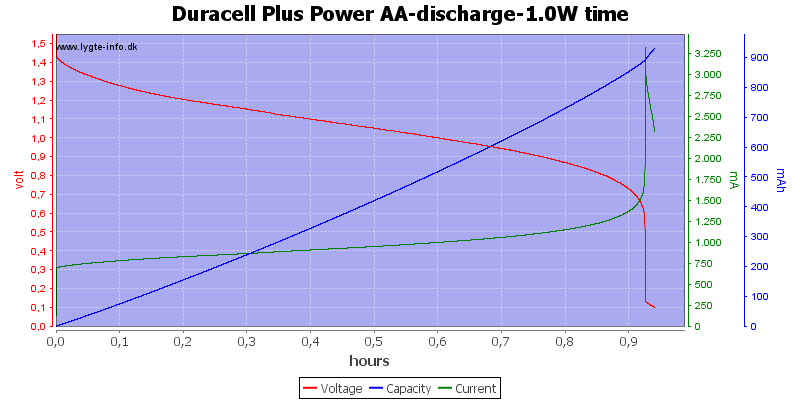
This is a very high load for an alkaline AA battery and it only lasts about one hour. Because I uses constant power draw the current goes up when the voltage goes down and this kills the battery very fast at the end.
My electronic load has some problems at the last part of the curve, it cannot handle 3A at 0.1V, the internal resistance and wire resistance is too large.
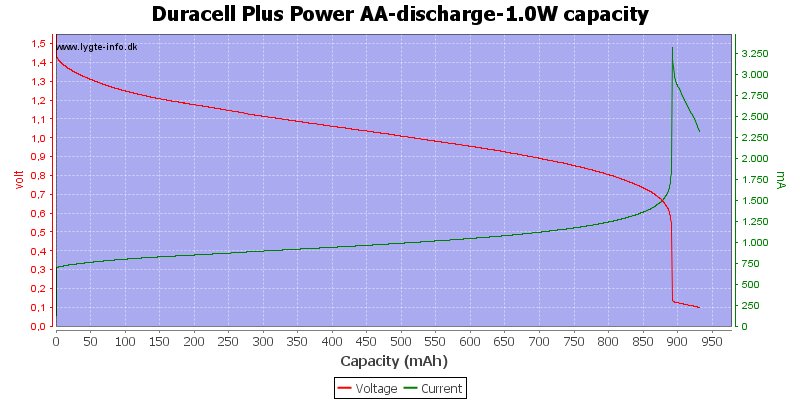
With this high load I only got about 900mAh from the battery.
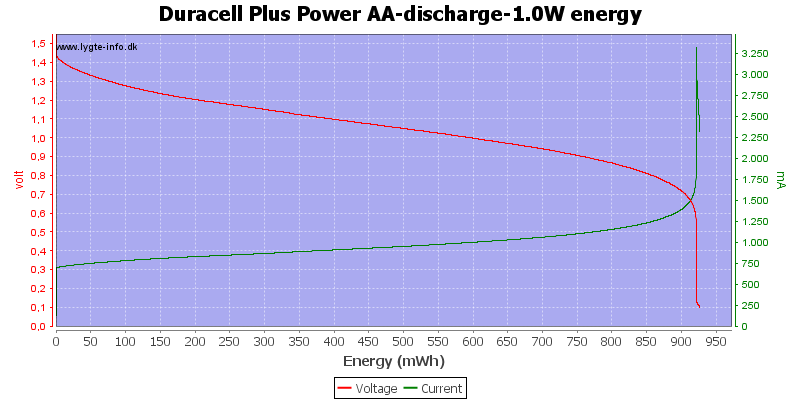
Exactly the same curve as above, I have just replace the scale again, this time to show the total energy deliveret from the battery.
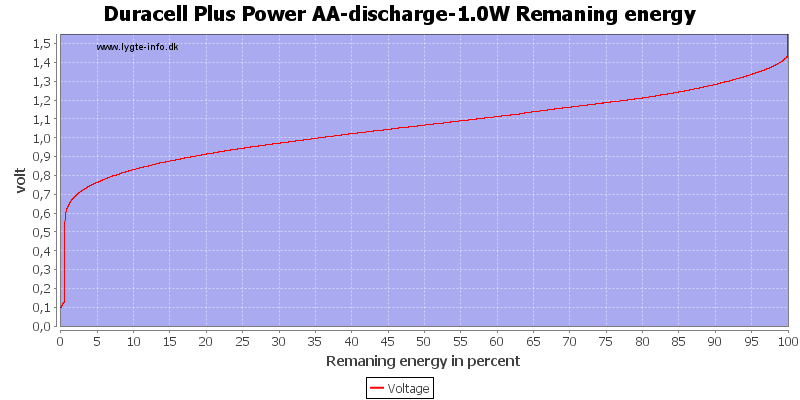
This can also be calculated as remaning energy in percent. Remember this curve is done while the battery is loaded in the equipment.
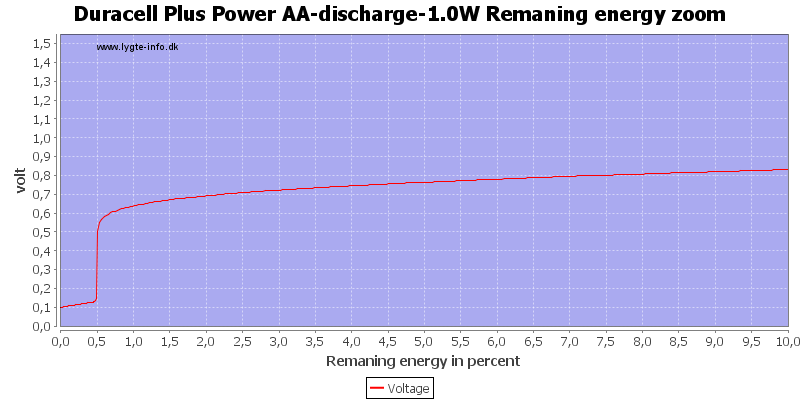
Due to the high current there is some energy remaning at 0.8 volt, but at 0.6 volt it is finished.
Test with 0.5 watt discharge
0.5 watt is 0.333A at 1.5 volt, but current will increase as the voltage drops.
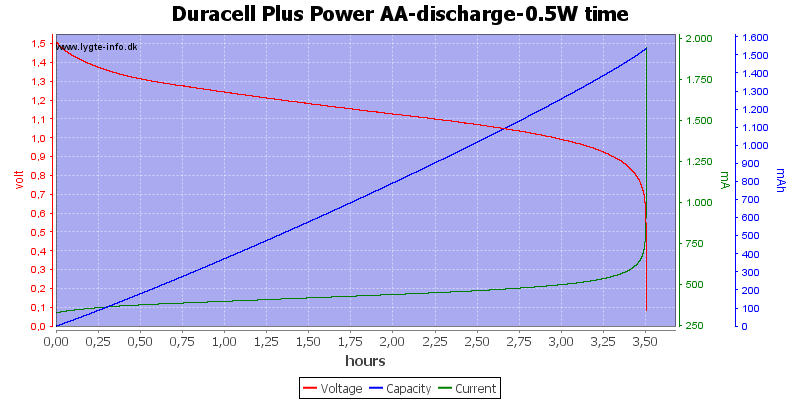
This is also a fairly high current for the AA battery, but not nearly as bad as above.
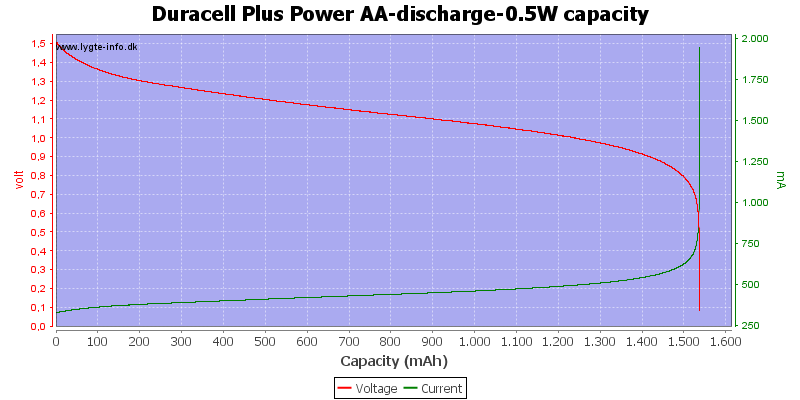
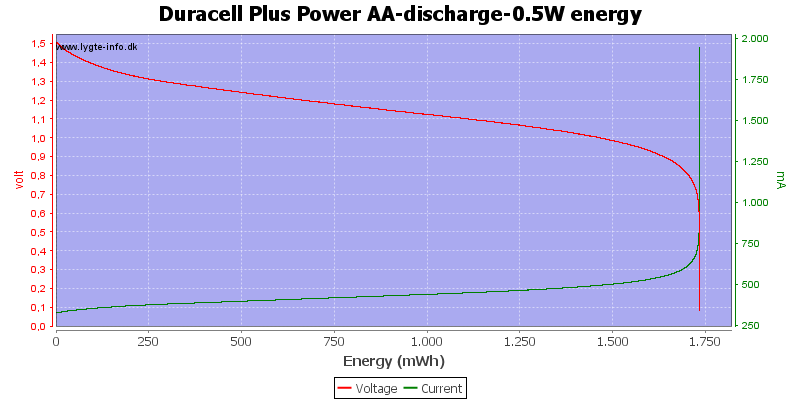
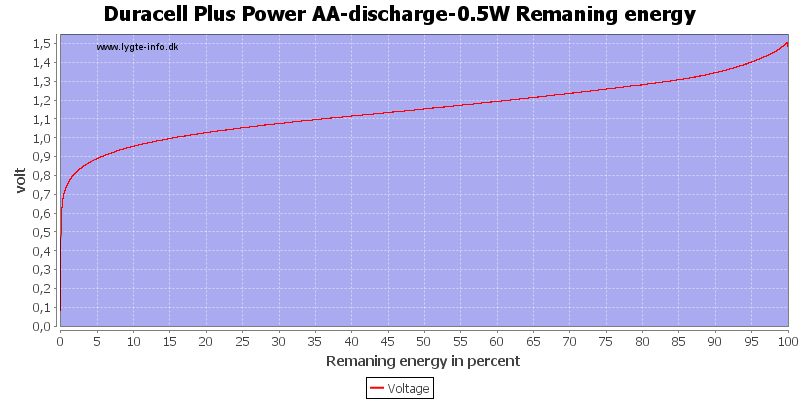
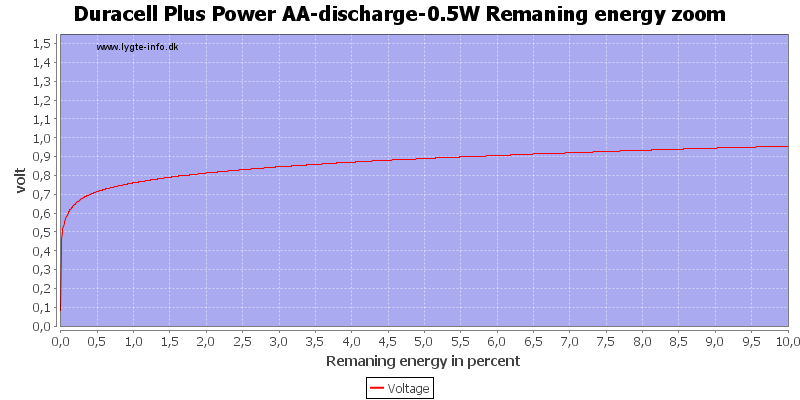
Here all the energy is used at about 0.7 to 0.8 volt.
Test with 0.2 watt discharge
0.2 watt is 0.133A at 1.5 volt, but current will increase as the voltage drops.
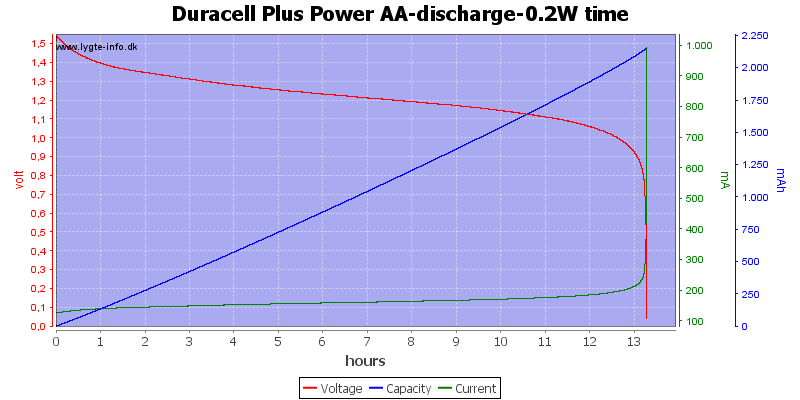
We are down in the range where alkaline batteries works.
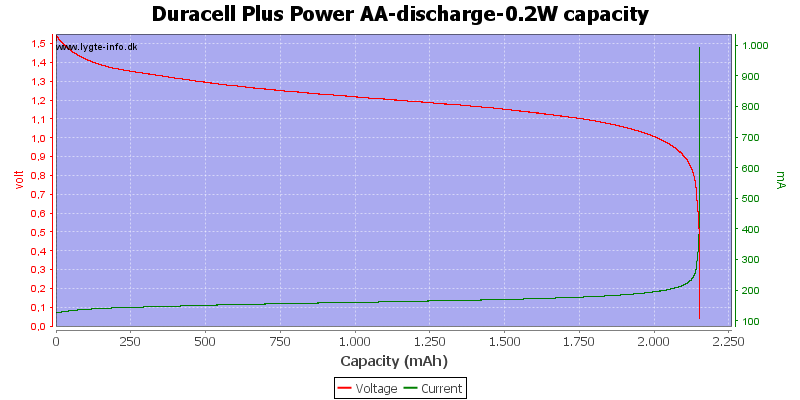
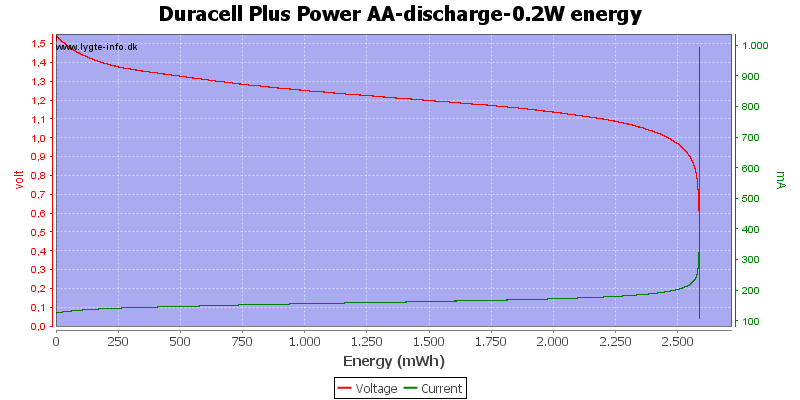
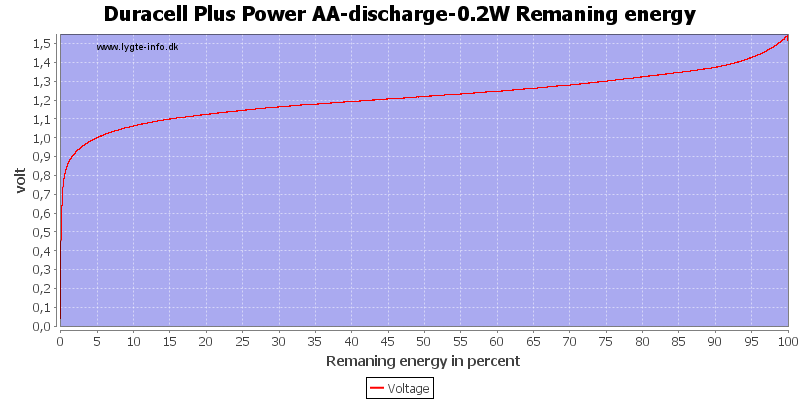
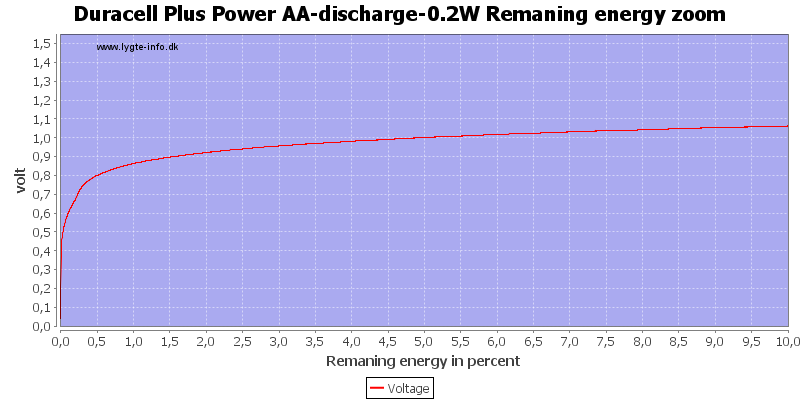
At 0.8 volt there is only 0.5% energy left.
Test with 0.1 watt discharge
0.1 watt is 0.067A at 1.5 volt, but current will increase as the voltage drops.
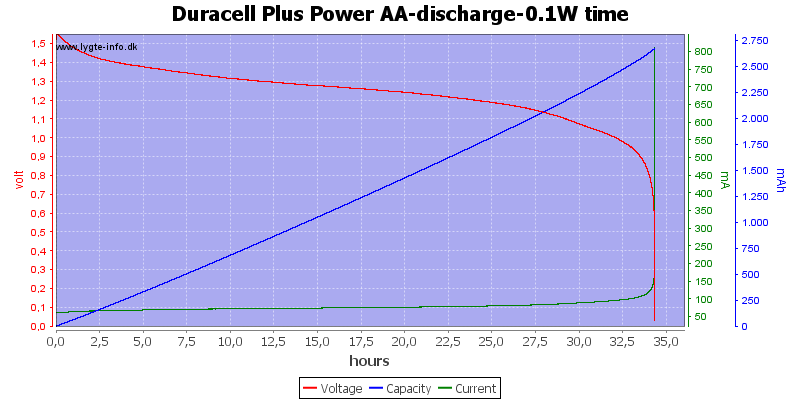
At this low power level they can match any NiMH battery in lifetime, but, of course, they can only be used once.
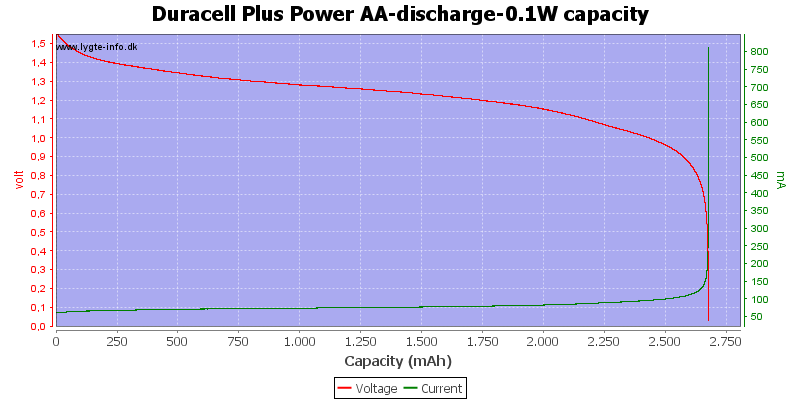
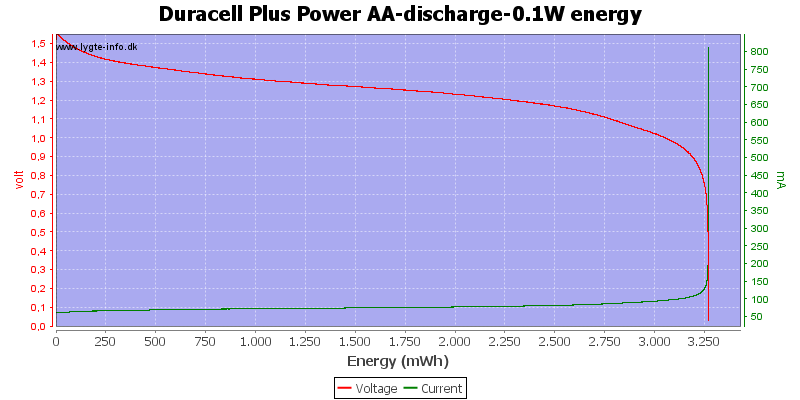
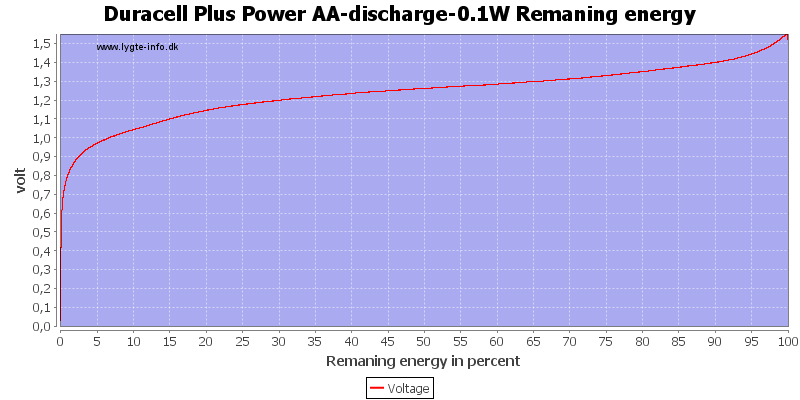
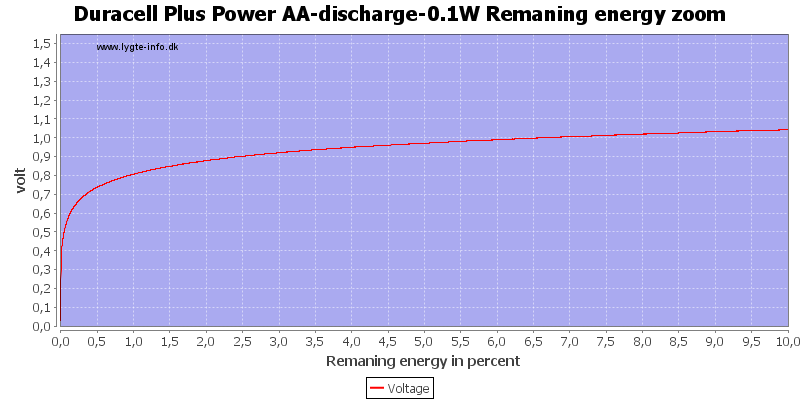
At 0.8 volt there is about 1% energy left in the battery.
Test with 0.05 watt discharge
0.05 watt is 0.033A at 1.5 volt, but current will increase as the voltage drops.
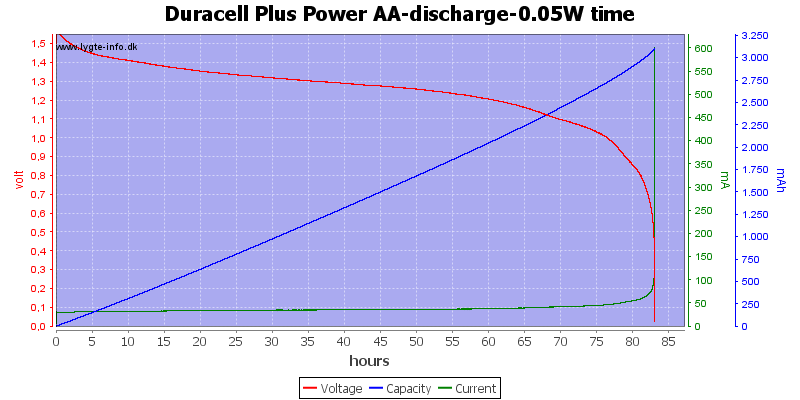
The voltage drop is slower with this low current, but the high current at the end will secure that the end is very abruptly.
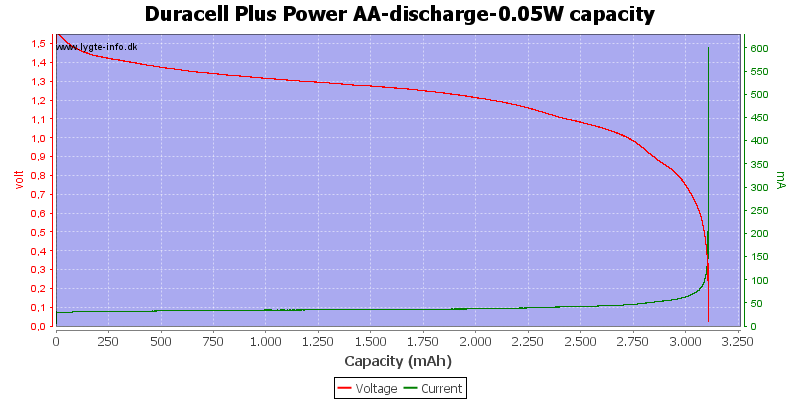
The capacity on alkaline is good at low currents.
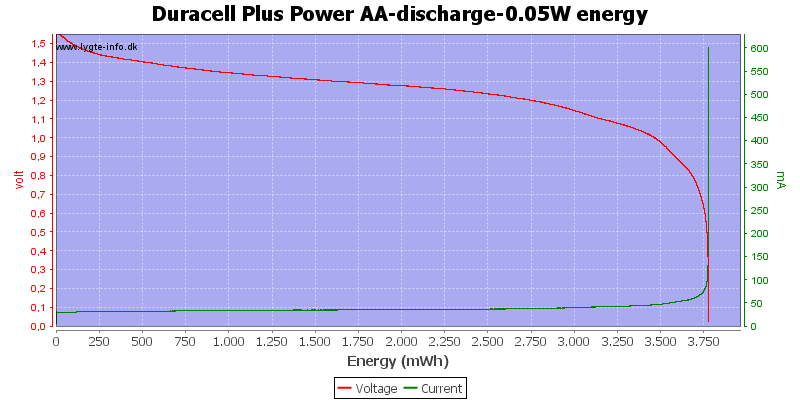
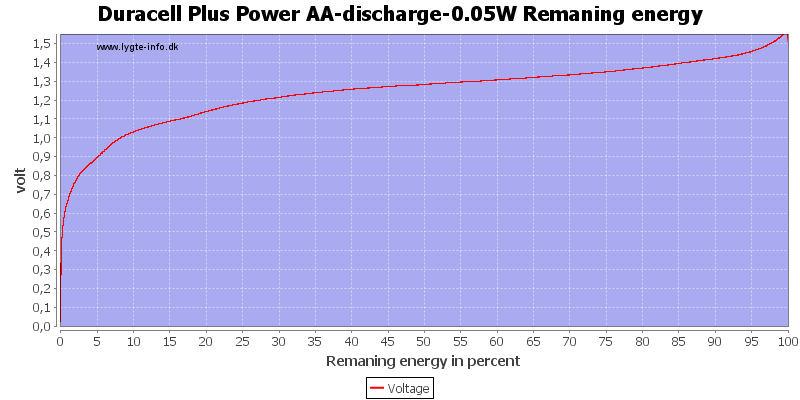
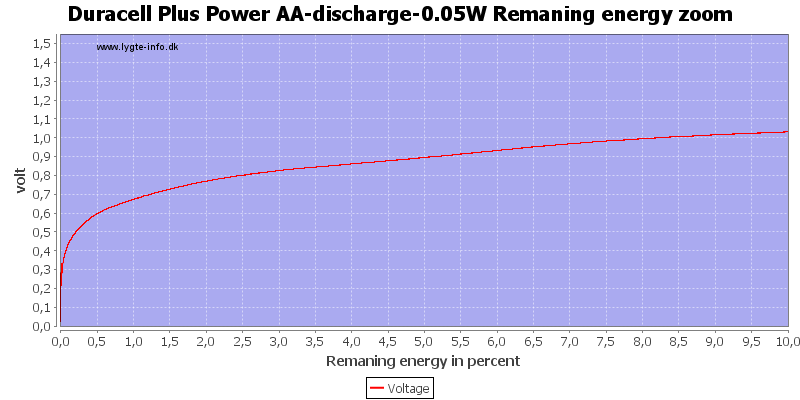
With low current there is some useable energy left, because the internal resistance is not as significant for the load.
At 0.8 volt it is 2.5%.
Measuring voltage
For the test below I had short breaks during the discharge. This shows how the battery voltage will increase significantly when the load is removed.
I.e. if you take the battery out of some equipment to measure the voltage, you will not measure the same voltage as the equipment see.
My breaks was only 1 minute long, this is not enough to recover all the voltage that the battery can recover, but it shows the principle.
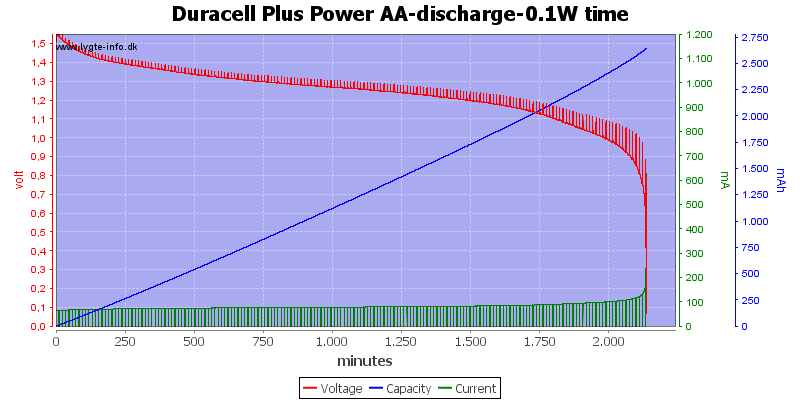
With a low load the difference between loaded and unloaded is not that huge, at least not with only one minute to recover.
But at higher load the difference is about 0.3 volt when the battery is nearly empty and that is before the battery starts to recover any voltage.
At the last pause the battery voltage is about 0.9 volt when loaded, but take it out and measuring it will show 1.2 volt and even more if it gets some time to recover.
The reason for the small voltage jump with a fresh battery and much larger voltage jump when it is nearly empty is the internal resistance. While the battery is discharged, not only does it loose energy, but the internal resistance will also increase, preventing high current drain.
Getting the remaining energy from a cell
At the current time (Aug 2015) there is a project on indiegogo for a device that can get the remaining energy out of a battery, it is called Batteriser.
The idea is to clamp a voltage booster around the battery to always get 1.5 volt from the battery, this can sound like a good idea and in a few cases it might be.
Lets look at the disadvantages first:
- Electronic always needs power also a booster and it is never turned off, this means the battery will be drained slightly faster. It will probably only be relevant for devices where there is years between battery replacement.
- Boosting voltage will loose some energy, at least 5%, but can be much more especially at lower voltages, i.e. using the remaning energy may mean shorter runtime than without using it, because the booster uses the energy.
- Because the booster always feed 1.5 volt to the device any battery indicator will show full, until the device dies due to empty battery. There will be no advanced warning.
- Devices that slows down when the battery is used, will not slow down and this means draining the battery much faster.
- Anything to clamp on a battery will make it larger, this will be a problem in some devices.
Where it is useful is for devices that need a high battery voltage to work, a good indicator will be if the device has problems running on NiMH.
Another way to get some more energy from drained cells is a "Joule theif", this is a low power circuit that can work at low voltage. This circuit is often used with led to make a weak light. A battery that has been used in a high drain device can usual power a joule theif for a fairly long time.
Generally any low power device that works at low voltage can be used to drain a battery completely.
Conclusion
Doing discharge curves down to 0.8 volt will give a small error in total capacity, but it is only a few percent.
If you can measure at what voltage a device stops working, use the above curves to see how much of the energy in the battery is left or use the table below.
I have collected all the results in a table:
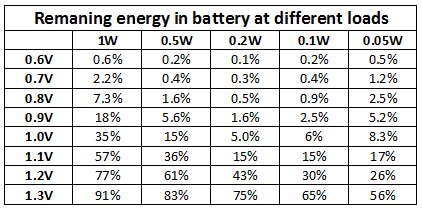
All these test at done with a constant load and without breaks. At higher loads taking breaks and/or moving the battery to a less power hungry device would have used more energy (I might look at this another time).
Notes
Standard battery test of Duracell Plus Power