We're now below 50% but it is still emitting light. I'll let it run till the cell protection cuts in. Now 47%
Still lit. Down to 29% Now 15,555 data points.
Looks like it'll still be lit when I go to work. It is just possible that hat eating won't be necessary.
Now down to 6%. Don't think I'm getting a whole day out of it.
How are you going to cook your hat?
Steamed....lol......with a little bbq sauce for good tasting. Thanks for doing the runtime....the light is still doing a good job at lighting up, have you tried turning the light off to see if it will come back on.
Came home to see to the hound - it is still lit. Now down to 2% after 28hr 20 minutes. It is about as bright as a sick glow-worm but it is still shining. Now 20,385 data points. This is impressive, I'd have thought the cell protection would have cut in by now.
That's a very impressive light then. And it sounds like most of the time the light was actually strong enough to be usable. But are you sure it has cell protection?
I'll save my dinner for another day....

The dropin won't have protection, but the cell does (or at least ought to have). Will see when I get home again in about 3.5 hours. There will be a lot of data in the graph. I have seen the protection circuit work in several of these Trustfire cells - couldn't say for certain about this one. If the voltage at the end is less than 2.5V I won't even try to recharge it. The protection circuit is supposed to cut in at 2.75V.
Once I get home, I'll try switching it off and leaving it for a bit before I try to turn it on again. Of course, it could be like some that will run, but not start when the voltage gets too low.
Ah, the CELL has protection? I didn't know that. My Akoray K-106 has an option cell protection feature in which it will not run when the current is too low, that's why I thought that it was up to the flashlight to protect the batteries.
The beauty of these lights is that the battery "rejuvenates" itself when it sits. (Maybe our in-house chemist could explain this Don?) So assuming that you were lost in the wilderness with only one battery, you could theoretically use the light all night long, then turn it off during the day, and it will turn on again the next day again at almost full brightness.
Well protection can go both ways.....the circuit on the cell will cut-off or the light will have one built in causing the light to turn off or give you a warning like blinking and such. If the light has built in low voltage protection then thats when i like to use unprotected cells.....nice to see that the the light is still going.
31.5 hours now. 1%. Dim enough that I can now look into the LED. Time to check the cell voltage. 2.68V which is after the cell protection should have kicked in. I'll let it stand for a few minutes then see what the voltage recovers to. 2.86V - even gives some usable light though I suspect, not for long.
Excel doesn't like time data that goes past 23hours 59minutes and 59 seconds. Obviously the next tick is 0 hours, 0 minutes and 0 seconds. This does make charting a bit tricky. I had just typed up a wonderful rant about this, but you are all lucky as I seem to have hit the browser's back button instead of posting it. Back to the fight with Excel - I feel much refreshed after a good rant about $£%^%$&*& Microsoft and its $£$@^%^$*&^*(&%^awful software. So back to the battle. It was still producing light at 31 hours 27 minutes and 6 seconds. There will be a runtime graph even if I have to download and install OpenOffice to do it. Excel 2007 and I just don't get on.
I once spent weeks trying to get it to plot 24 hour probability density functions for various revolting medical and budgetary reasons. It can't so probability density functions and my knowledge of Mathematica (which can do such things) is rather less than my knowledge of the dynamics of galaxy formation. That question in the exam paper earned me exactly 0 marks and a note at the bottom saying, "I suspect you weren't at the lectures". Sad bit is - I was at those lectures.
At long last, the runtime chart on low. It runs for a very, very long time on low. I gave up at 31hr 27 min 6 sec as I was concerned that the low voltage cutout on the cell wasn't working. Fortunately the cell recovered and is on charge now. Conventional reckoning is that 50% = runtime so it has a runtime of 20 hours and 15 minutes. It'll actually produce useful light for something like a day on a 2400mAh (Probably optimistically rated) cell. It will produce some light for another 14+ hours. Given that hardly anyone is ever in darkness for more than 24hr at a time and it is dark here for about 16 hours a day but I'm asleep for 7-8 of those, I should get 3-4 nights of usable light out of it. Ignore the spikes at 10 and 14 hours - was looking under the desk with a bright light.
So, without farther ado, here's the runtime chart.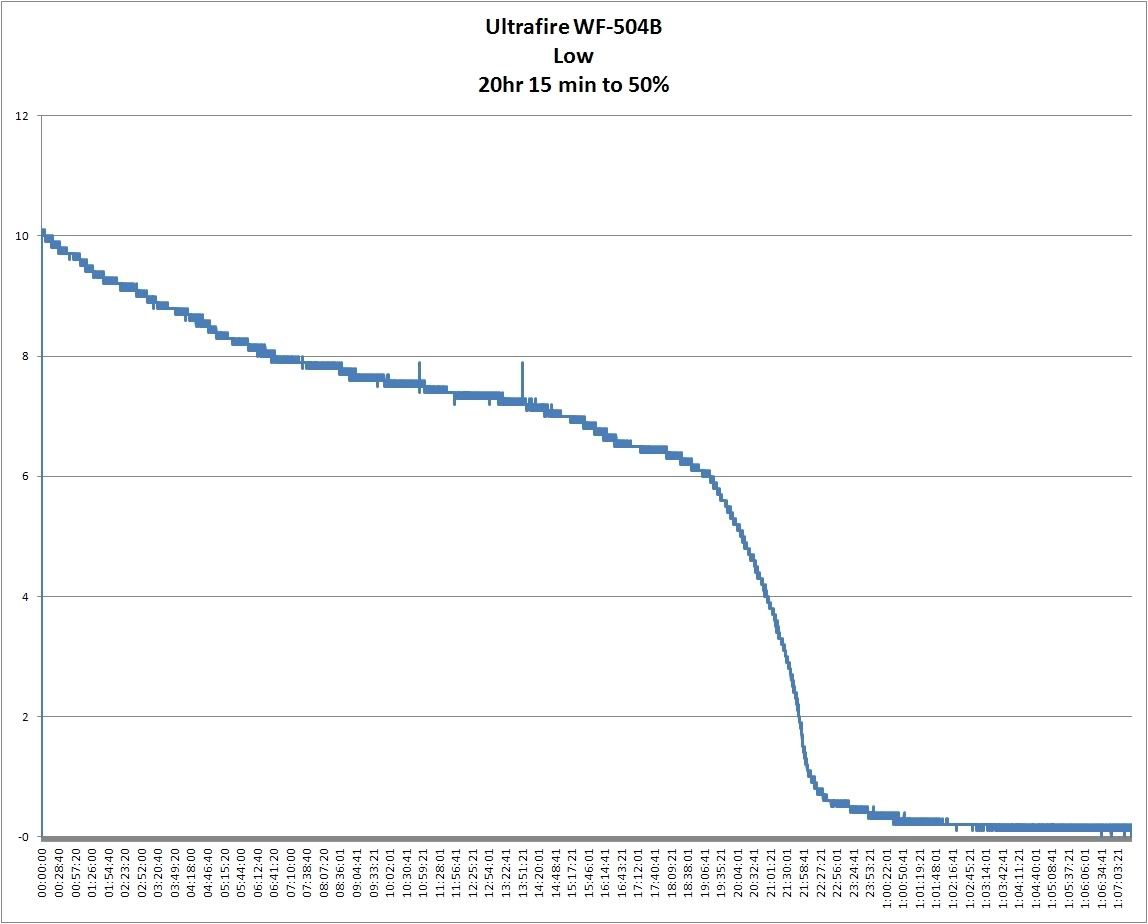
Very nice! I fixed the size for you, the height was at "80" instead of blank.
So as far as I can see, it produced very usable light all the way to about 22 hours. Very impressive.
All cells will to a certain extent recover on resting. What follows is a gross over-simplification but how we get electricity from a cell/battery is as follows.
A chemical reaction takes place in which something is oxidised and therefore loses electrons. These electrons can do work as the energy of the chemical reaction pushes them around - the force of that push is what we call voltage.
In a cell that can be recharged, the reaction is to a greater or lesser extent reversible - by shoving electrons back in we can "undo" the chemical reaction.
The substance that is being oxidised has to move inside the cell in order to react. When it gets too crowded round the anode, the reacted material has to move away to let fresh material get there to react. It takes time for this to happen - especially as the ions' paths in solution are essentially completely random. Think of a rush hour where every driver is drunk and you get the general idea. Once they sober up, to push the analogy way too far, things get a bit better organised.
So when the battery gets a rest it can organise itself a bit better and there are more ions at the anode ready to push.
The details of the actual construction of modern cells are incredibly complex - there is a whole lot more to the story than that. Wikipedia's stuff on the various battery types and how they work is actually pretty good.
Remember, I last taught chemistry in 1986 so I'm more than a little out of date and my memory has never been that good.
Very clear, thanks Don!
Now that is a very long time to find another battery......especially if your in the boonies.
Even in a place with no power (where you'd probably run it on low nearly all the time) you'd likely only need a cell a week - allowing 2 cells a week you'd likely be fine. And that'd allow a fair amount of using the throw and more power. In Shetland (where I worked in 1989) you'd need more. At this time of year you need artificial lighting just about all the time, it never really gets light in winter. Or dark in summer.
I note some of the more expensive torches boast regulation.
The effect of regulation is that the LED supply voltage is closely controlled at a level somewhat below what the batteries can deliver when (say) 50% discharged.
I don't know how this voltage is decided, particularly if different batteries are to be used, but I suppose the specs call up a specific battery type with known discharge rates.
The thing I don't understand is how can torch LED's stand the unregulated battery voltage variation as the batteries discharge?
The conventional wisdom is that a current limiting resistor is used which I suppose is a poor mans regulation.
Even so the reported differences in brightness over a supply current range varying by around 100% (0.9 amps to 0.5 approx) do not make much sense, it's almost as if the higher current is "wasted". Do we have an electronics specialist who can shed an accurate insight on this?
Peter O
Thanks for the review: Can the drop-in included with this light be run off of 2xAA lithium cells?
The quoted voltage range is 2.7-4.2V
Depends how much the voltage drops under load. If it does, I'd not expect it to run for all that long till the voltage dropped below 2.7V. It also depends on how the driver handles low voltage. Since it is intended for use with a single 18650 and 2.7V is the voltage at which protection circuits are supposed to cut in, it may be that it will run happily to below that. Or it may be that it will cut out at that voltage to prevent the cells from becoming over discharged.
I will drop it into an L2r and try it with alkalines and NiMH tonight to see what happens. Lithium primaries here are considerably more expensive than genuine Eneloops so I don't use them.