OK, this is something which I can potentially provide some helpful info: I am a biochemist whose work deals a lot with fluorescent materials (including a number of fluorescent proteins).
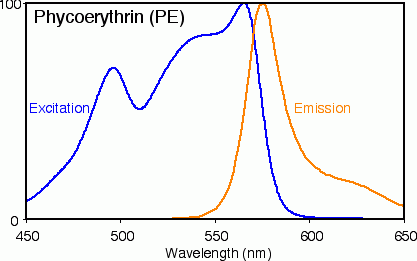
A bit of background for those trying to help, it is not uncommon for highly efficient fluorescent proteins to only have 5-20nm of difference between peak absorbance and peak emission. The image below is the absorbance and emission spectrum for a common fluorescent protein Phycoerythrin: Ab(max)=565nm and Em(max)=575nm. Along with this close relationship between the absorbance and emission, you can see there is also a rather steep roll-off when it comes to absorbance to the red side of the peak. As such, light which is 10nm from Ab(max) is almost entirely unabsorbed.
Here is my recommendation:
Understand your absorbance curve for the biofluorescent proteins and use it to your advantage. While the above image has multiple peaks, even if your protein of interest only has one peak, it will have its sharpest roll off in absorbance to the “red” side of the absorbance curve. This means the “redder” the light is, the less it absorbs. Your focus on the FWHM of the LED is giving you only a part of the picture, as there is more than one way to target this mismatch. For example, targeting bluer wavelengths with wider emission FWHM might provide you with a better match (say 440±20nm).
Additionally, if you want optimal performance then you are going to HAVE to use filters to ensure the light you are capturing comes only from the fluorescence you are targeting. Typically, we use a combination of a Band Pass filter on the excitation source combined with a Long Pass (aka High Pass) and a Band Pass filter on the emission detection side to ensure that all excitation light is excluded and we are ONLY capturing the fluorescent emission.
An example filter set which would result in your excitation source illuminating only within 400-450nm and the image sensor only collecting light between 460nm and 480nm. Note that these are research grade and aren’t exactly cheap:
Excitation Filter (425/50): AT425/50x | Chroma Technology Corp
Emission Long-Pass (460nm): ET460lp | Chroma Technology Corp
Emission Band-Pass (455/50): ET455/50m | Chroma Technology Corp
In your case, it appears you have multiple emission wavelengths in which you are interested (side note: that yellow-orange glowing newt likely has another absorbance peak around 500-540nm) so you might need more than one filter set or just drop the Band Pass filter on the emission side. That being said, you typically want at least 5nm separation between your excitation filter and your emission filter.
Feel free to ask me any questions!



