Hey BLF,
Here’s a very informative video about electropolishing aluminium.
Anyone wanna give it a try?
Hey BLF,
Here’s a very informative video about electropolishing aluminium.
Anyone wanna give it a try?
Hmm interesting. Polishing by hand is a royal pain. I’ve polished motorcycle parts on a buffing wheel and it’s a laborious process.
Shiny and flat are two very different things. That method might make it pretty but does nothing for altering the surface finish in the way mechanically polishing does.
I'll give it a try when I have better equipment. I'm keeping an eye out for a surplus hot plate with magnetic stir capabilities at the local university, and where my sister is a chemistry grad student.
This process might help save damaged reflectors, and would probably be best with finishes that have gone dull rather than scratched. I'd also like to see if it can be followed up with aluminum electroplating, although I suspect electroplating will mostly result in build up along the edges like it does with copper electroplating, but there's another technique I've been playing with that may result in a much more even coating.
cpfdaniel calls it electroplating below, and it does appear very similar, although the strongest acid I currently use is acetic acid that says "white vinegar" on the label.
I would call this electroplating rather than polishing. There is no polishing happening in that jar.
At 4:40, you can see what’s going on on a micro level. I don’t think there’s any plating going on, but the rough surfaces with micro bumps and ridges are getting worn away faster due to larger surface area per volume compared to flat areas.
It's just removing the oxide layer on the surface which isn't the thing you're trying to achieve by sanding & polishing. Shiny doesn't matter, shiny just happens to be a side effect of first making it flat and then removing the surface scratches.
Removing the oxide layer is useful for me. It'd help with electroplating.
I am interested. If it works, it would be a boon to making my DIY reflectors. I will investigate in the coming months. I have everything I need to do it with the exception of the phosphoric acid.
My To-Do list gets longer by the day...
A little bit of research makes me optimistic that this is a real deal process...for stainless steel. There is even an ASTM standard for stainless (ASTM B912). Further research shows that it is used for aluminum as well.
Before and after electron microscope pics from ableelectropolishing.com.
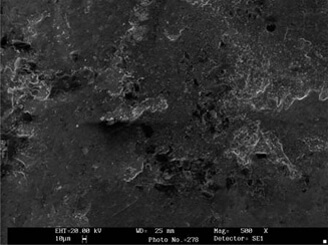

There seem to be at least two theories as to why this works. Neither one of them make much sense to me.
There seem to be at least two theories as to why this works. Neither one of them make much sense to me.
- The surface area of protrusions is greater, thus they are "reverse plated" at a faster rate than the rest of the surface. I discount this theory because the pits would deepen more quickly too.
That part makes sense. I think I said something about it in the electroplating thread. There's a similar (or same?) process that cycles in a short reverse current. Sharp areas plate and deplate faster than the micro depressions between, so each cycle results in shallower depressions.
I think your second theory is a little off. Reverse plate is deplating. No plate is added to that surface during the reverse plate.
Typing this out is making me realize I need to add some reversible switches to my electroplating kit.
First. Neither of those theories are mine. I'm just regurgitating them as food for thought.
Next, I think that you missed my point in #1 above. The protrusion is removed because it has more surface area than the flats. Well, so do depressions. So the areas of greater surface area would have material removed more quickly than the flat surface...both the protrusions and depressions would lose material more quickly...ie the pits would get deeper just as fast as the peaks would be removed.
Last, I'm not sure where you read that I say anything is added. What I wrote is poorly worded, but that's kind of how I found it written. I guess I should have said that the thinner layer at the protrusions allows more current to flow to the protrusions thus removing them quicker while the thicker film at the pits insulates the current, protecting it from the process. "Reverse plated" is how one of the companies that do electropolishing refer to it and I think it's an apt description for how material is removed in the electro-chemical process.
Anyway, I like to know why things work but if I can't find out why and it still works I'll be ok with it. For instance I'm good with gravity and no one really know the mechanism for how it works. I've now read enough about this to acknowledge that it is a real process that works even if I don't know why.
First. Neither of those theories are mine. I'm just regurgitating them as food for thought.
Good to know.
Next, I think that you missed my point in #1 above. The protrusion is removed because it has more surface area than the flats. Well, so do depressions. So the areas of greater surface area would have material removed more quickly than the flat surface...both the protrusions and depressions would lose material more quickly...ie the pits would get deeper just as fast as the peaks would be removed.
I believe you understand the result, but not why it's happening. The protrusions react faster. It's hard for me to explain, but it's like they shield the parts in between or that they have the opportunity to react first, perhaps a bit of both. Electricity reacts with the edges and high points more than the low spots. Lightning is the best analogy I can think of.
Last, I'm not sure where you read that I say anything is added. What I wrote is poorly worded, but that's kind of how I found it written. I guess I should have said that the thinner layer at the protrusions allows more current to flow to the protrusions thus removing them quicker while the thicker film at the pits insulates the current, protecting it from the process. "Reverse plated" is how one of the companies that do electropolishing refer to it and I think it's an apt description for how material is removed in the electro-chemical process.
Okay, I think we both agree that reverse plating means the same thing as deplating, at least for the surface of the work piece. I did misunderstand your intention there.
We're both trying to learn here. If you're taking it personally, I apologize for unintentionally putting you on edge. Anyhow, I don't agree that a thicker layer provides an insulating effect. It's the protrusions that matter. Let me give two examples.
If an aluminum work piece is attached to one electrode, and another piece of aluminum is the other electrode, it's okay for the protrusions to be removed in the reverse plating process. The reverse plate step, or what I was calling deplating, could be used from beginning to end. The problem with that is it only removes material. I believe you agree with this, even if we don't agree about the why. Since you mentioned reflectors, this is probably all that would be applicable to you. Understanding the "why" may not be that important.
Things are a little tricker if the work piece that's connected to one electrode is a different type of metal than the metal connected attached to the other electrode. The electrolyte should have the ions of one metal, but not both, or the electrolyte is fouled. So the forward plating process would have to be first, but it's critical that the reverse plate period not be longer than it takes to remove only the plated material. Alternating between plating and reverse plating will reduce lost material, and might even have a net addition of material. When the current switches direction from reverse plating to plating, it will replate the protrusions more than the areas between, but the plate on the protrusions is removed much faster than in the depressions during deplating, enough so that the reductions of the protrusions and additions to the depressions should result in a flat surface when this cycle is repeated enough times. Since right now I'm plating one metal onto a different metal, and then a third metal on top of that, this is more applicable to me at the moment. Understanding the "why" must be good enough to avoid fouling the electrolyte with ions of both metals, and especially not the ions of metals in the first and third layers.
Anyway, I like to know why things work but if I can't find out why and it still works I'll be ok with it. For instance I'm good with gravity and no one really know the mechanism for how it works. I've now read enough about this to acknowledge that it is a real process that works even if I don't know why.
Fair enough. As long as you stick with using the same metal on both electrodes and know that you may need a deeper understanding if you work with two or three different metals.
I didn't take it personally at all. It's just a discussion. No apology necessary. I, however am sorry if I came across as angry. My co-workers often make fun of me for my...lack of tact.
I agree with your lightning analogy. In fact, I was thinking of it that way (sort of). When I do anodizing, the ano has difficulty in deep(ish) grooves due to the more direct path the electrons can take to the closer metal at the surface. The only trouble I have with the lightning idea is that we are only talking about very small differences in height between the peaks and valleys (<.002") for electro-polishing versus 75x that for anodizing something that is grooved.
Here is a picture showing what we are talking about.
This is the best explanation that I have found and bolsters your argument.
http://www.electropolish.com/basic-principles/
When I attempt this I will use aluminum for the cathodes and see how it goes.
What do you think of the guy in the video using SS for the cathodes? Also, one of the commercial companies that do this do it with cathodes that closely match/mirror the shape of the piece that they are electro-polishing. Beneficial maybe?
My addled brain has no idea how this works, however at the top of the peak there is less material being attacked from all sides, whereas at the bottom of the valley there is more material being attacked from only one side, so while all of the surface is being deplated at the same rate the peaks will disappear faster than the valleys.
Cheers David
We're okay. Maybe quite similar as far as tact goes. At least I despise the effort it takes to have it.
I didn't realize deep grooves were a big problem with anodizing, at least as far as electricity is involved. I haven't been doing it like you have, but I thought the main issue there was agitation to force the solution into the grooves. Have you figured out a way to deal with those grooves? Did you post more anodizing videos? I only saw the one where your neighbor walked up and you told him your son wanted a purple light.
I like that link. It's almost exactly how I was thinking of it, although current density is a different explanation as to why the electricity goes where it goes. That matches some other stuff I think I know though, so I'll accept it until some book reading tells me different.
Good question on the SS. I wonder if that's done because aluminum bonds poorly with SS...and I'm only guessing that that bond is poor. I've heard of lead being used for kind of the same reason, but mostly because it doesn't put ions into the electrolytic solution. I'll have to look into that.
The section in that reference made me think about alloys. I wonder if the pitting in the video was because the magnesium and copper was reverse plating faster than the other metals in the alloy? That would be another kink in trying to achieve a great finish. If only we could do aluminum vapor deposition. That seems soo much easier, but expensive. Yesterday someone mentioned a kickstarter for modders. How about we do a kickstarter for a blf vacuum deposition kit? We'd need to raise about a million dollars. That's my modding fantasy. Getting to a point where I have personal use of a lathe, mill and cnc is attainable, but that vapor deposition is too far out of reach.
The closely matched cathode is one of the solutions I read about to solving uneven plating, or in this case, reverse plating. Moving the electrodes or using multiple electrodes is supposed to help too. Closely matches cathodes is impractical for me right now, so I'm going to try playing around with placement of multiple electrodes.
There's another process I've seen called electroforming that you might be interested in. I think it's basically electroplating until you have a solid part you can remove from a mold. Can you machine glass on a lathe? I think that would work well as a reflector mold, and then hit it with a flame to smooth the surface. There are ways of electroplating glass, so I don't see why it couldn't be continued into electroforming.
Earlier I mentioned a hot plate with a magnetic stirrer. I just found this.

Magnetic Stirrer W/ Hotplate for <$30: A magnetic stirrer with a hotplate allows you to mix and heat solutions with ease. These are commonly used in chemistry classrooms and cost upwards of $160! But I'll show you how to build one for less than...
~$30. That could save me a LOT of money. Time too since I've been having a hell of a time finding a deal on a hot plate.
Its important to always be tactful you ninnies. :bigsmile:
Alas, glass cannot be machine on a lathe as far as I know.
As far as vapor deposition goes, I have thought on it. I wonder what the least vacuum one could get by with would be. I can easily make the vacuum chamber with an electric heating element. It's just the deep vacuum required that has held me up (4-5 micron is stated as required in some places). I've read that the depth of vacuum affects the coating of irregular surfaces and the time required to coat. Reflectors don't really have irregular surfaces and time is less of a concern to me than it would be for a company trying to make a profit. If a less deep vacuum could be used 2 stage vacuum pumps for heating and air conditioning are not that expensive and can get down to 15-25 micron vacuum. I'm almost willing to make that bet.
Oh, and I have not made any more anodizing videos since it is rather boring (at least to me).
I wish my son was awake. The light was for my daughter, but it will be fun telling him that you (and the whole internet) thought he wanted a girly purple light!
I have not really tried to anodize grooves since the first try. I should do an experiment to see what works and what doesn't. I'm sure current density plays a factor as well as agitation.